|
AIDS Therapies
Introduction
There is no cure or preventative vaccine for AIDS
|
Today much research is devoted towards the discovery of new drugs for treating people infected with the human immunodeficiency virus (HIV), the virus that causes AIDS. This research focus on AIDS is quite warranted, for the spread of this deadly disease is reaching catastrophic proportions. A solution to the AIDS epidemic is one of the world's top priorities, yet like cancer, is also one of our greatest challenges.
Unfortunately research has not yet yielded a cure or preventative vaccine. However, tremendous strides have been made towards controlling the virus and slowing or halting the progression in patients from HIV infected status to having AIDS. Additionally, because scientists have struggled to develop satisfactory drugs for use by the doctors to treat their HIV-infected patients, the medical profession has learned a great deal about how underpowered drugs can be utilized in creative ways to significantly increase their efficacy.
HIV Drug Targets
There are a number of stages within the HIV lifecycle that researchers have targeted as potential weaknesses of the virus to be exploited with potentially effective therapeutics. Generally, when looking for a viral Achilles heel to target with a drug, scientists look for essential viral processes that are catalyzed by a single enzyme. Also important is whether this enzyme is unique to the virus, for if there is no similar human enzyme, then a drug that targets this viral enzyme might have very low side effects and toxicity when administered at doses high enough to eradicate the virus. Promising pharmacological targets for inhibition include the virion binding and fusion, viral replication, integration, proteolytic maturation, and virus-specific transcriptional regulation.
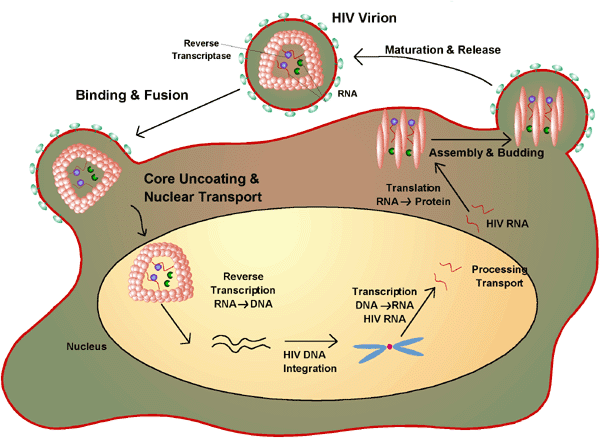 |
 The HIV Lifecycle The HIV Lifecycle
|
Virion Binding
The first step in the HIV infection of a cell is the binding of the virion particle to proteins on the surface of lymphocytes. This initial interaction is mediated by the HIV envelope protein (GP120, or ENV), which targets the lymphocyte's CD4 receptor. Blocking this interaction has obvious benefits to interfering with HIV infection.
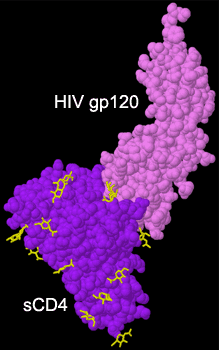 |
 HIV ENV complexed with sCD4. HIV ENV complexed with sCD4.
Click on the graphic to manipulate the structure in 3D.
|
The virion binding event has been targeted by AIDS researchers. One therapeutic approach taken has been to administer a recombinant soluble (non-membrane bound) form of CD4 (termed sCD4), with the hope that it would bind and occupy all of the viral envelope binding sites, thus interfering with the virus's ability to bind to lymphocytes. Unfortunately, drug trials with sCD4, as well as a few more creative derivatives, proved this treatment to be ineffective.
Investigators have not given up on the idea of blocking the early virion binding event. New biochemical advances such as the crystal structure of the HIV envelope protein bound to CD4 have allowed researchers to map the important contacts for the CD4-GP120 interaction. Using structure-based methods of drug design, small molecules can now be developed to interfere with complex formation. Additionally, it has become clear that upon binding CD4, the viral envelope protein undergoes conformational changes that open up a secondary receptor binding site. These secondary host cell surface proteins are called coreceptors. The structural picture of this important interaction, along with this recent information that HIV binds to two cell surface receptors in a sequential process, has inspired many scientists to again target virus-cell binding for the development of an effective chemotherapy for HIV.
Replication
HIV is replicating very actively at all stages of infection, yet is kept in check by the mobilized forces of the immune system. The first efforts to design an effective therapy for HIV infection targeted viral replication because any inhibition of replication would likely substantially reduce the amount of virus floating around in the body (viral load). This approach has shown promise, for drugs targeting replication lower the viral load of infected individuals. Fortunately, HIV patients whose viral load is low appear to take longer to progress into AIDS.
HIV replication is catalyzed by the viral enzyme reverse transcriptase (RT), which copies the virion RNA genome into DNA. This first class of drugs that target replication are called RT inhibitors. They are the most prevalent of HIV drugs, and include the first drug used on a large scale to treat HIV, AZT. The RT inhibitors are divided into two classes, nucleoside analogs and non-nucleoside inhibitors.
Nucleoside Inhibitors of RT
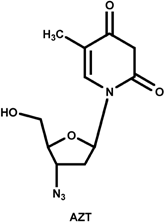
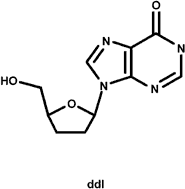
The nucleoside analogs are not traditional enzyme inhibitors in the sense that they do not function by binding tightly in the active site, competing with the natural substrate and inhibiting the chemical mechanism of the enzyme. Instead, they are modified nucleoside bases that lack the 3'-hydroxyl group that is used by the polymerase as it adds nucleotides during replication. As RT copies the viral RNA genome into DNA prior to integration, these nucleoside analogs are incorporated into the growing DNA strand, terminating replication prematurely. They work exactly like the nucleotide reagents used in Sanger sequencing of DNA. Three common nucleoside analogs are AZT, ddI, and 3TC. All of these inhibitors are structurally similar to one of RT's natural substrates for replication, deoxynucleotides, and all possess a missing or substituted 3'-OH. The nucleosides analogs are not phosphorylated intentionally for delivery purposes, for the charge of a triphosphate would preclude them from passing through the hydrophobic cell membrane. Once inside the cell, the three phosphates needed to make them effective substrates for RT are added to the 5'-OH by cellular enzymes.
 |
 |
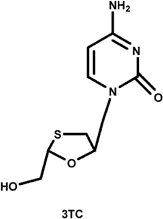 |
 The commonly prescribed HIV RT nucleoside inhibitors AZT, ddI, and 3TC. The commonly prescribed HIV RT nucleoside inhibitors AZT, ddI, and 3TC.
|
Non-Nucleoside Inhibitors of RT
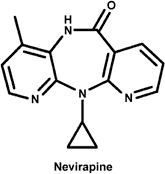 |
 The common non-nucleoside HIV RT inhibitor Nevirapine The common non-nucleoside HIV RT inhibitor Nevirapine
|
The non-nucleoside inhibitors are non-competitive or mixed inhibitors of RT. They bind to a hydrophobic pocket on RT proximal yet separate from the active site that is only present upon binding of nucleic acid substrate. Because these inhibitors do not bind to a site on RT that is of critical functional importance (like the nucleoside analogs in the active site), mutations in the binding sites for these inhibitors are readily selected. Resistant strains therefore emerge quickly in patients treated by this class of RT inhibitors, generally in as short as two weeks, greatly limiting their therapeutic utility. Nevirapine is a commonly prescribed of this class of RT inhibitors. Note that the non-nucleoside inhibitor pictured bears no resemblance to substrate RNA or nucleotide.
Integration
After entering a cell and copying its RNA genome into DNA, an essential next step for HIV is the integration of viral DNA into the host cell's genome. In a process unique to retroviruses, the viral DNA moves into the nucleus and inserts itself fairly randomly into the host cell's DNA. The integrated viral genome as DNA is termed proviral DNA. Because integration is catalyzed by the virally encoded protein HIV integrase (INT), which has no host cell homolog, integration is an attractive target for chemotherapy.
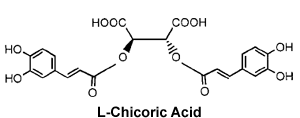
A number of compounds have been identified that inhibit the HIV integrase. Many of these are intercalating agents which bind to DNA by slipping between internally stacked bases. One concern regarding the use of all intercalating agents for treating patients is that because they disrupt the DNA structure, they are likely to be carcinogenic. In addition, these intercalating inhibitors generally lack the specificity required for an effective antiviral therapeutic. Other INT inhibitors may inhibit integrase directly, such as L-chicoric acid, and even bind HIV integrase in the active site, which is witnessed for the new integrase inhibitor 5CITEP.
 |
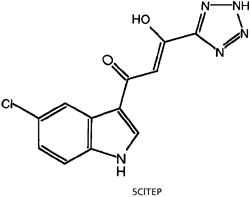 |
 Potential lead compounds for an effective HIV integrase inhibitor, L-chicoric acid and 5CITEP Potential lead compounds for an effective HIV integrase inhibitor, L-chicoric acid and 5CITEP
|
Another promising integrase inhibitor that is currently in clinical trials is Zintevir. Interestingly, this drug is novel in that it is a simple oligodeoxynucleotide with the sequence:
5'-GTGGTGGGTGGGTGGGT-3'
Viral RNA Synthesis and Processing
HIV RNA production is catalyzed by the host cell transcriptional machinery
|
HIV RNA is synthesized by transcription from the proviral template, which mimics a cellular gene, using the host cell transcription machinery. Transcription of HIV RNA is catalyzed by host enzymes that are essential for normal cell function, including cellular RNA polymerase II. Therefore designing specific inhibitors of viral RNA synthesis is difficult. However, HIV does encode some of its own transcriptional activators, namely the HIV protein Tat, which might serve as specific drug targets for combatting the virus. Tat is a very strong activator of transcription, and is essential for viral propagation, yet no drugs that interact with Tat have been shown to be effective antiviral compounds.
Following transcription in the nucleus, viral RNAs are processed and transported to the cytosol in a highly regulated manner. HIV Rev is the viral protein responsible for this regulation, but to date no therapies have successfully targeted this protein.
Proteolytic Processing
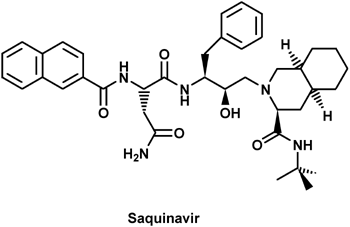 |
 The effective protease inhibitor Saquinavir The effective protease inhibitor Saquinavir
|
In the mid-1990's a new class of promising HIV drugs emerged which target the proteolytic maturation process of the viral lifecycle. HIV proteins made inside infected host cells are produced as large polyproteins, not yet capable of arranging into an infectious virion particle. HIV encodes its own essential protease (PR), which during the later viral lifecycle stages of assembly, budding, and maturation cleaves these precursor polyproteins into the smaller proteins found in mature, infectious virions .
Because of its central role in the viral lifecycle, the protease is a favored target for ongoing HIV drug development. A general rule for drug design is that inhibitors possessing tight binding in the targeted enzyme's active site make good chemotherapeutic agents. The best of these tight-binders are transition-state analogs which mimic the strained geometry of the substrate midway through its conversion to products. Note from the illustration that Saquinivir, as well as many of the other HIV protease inhibitors, is an oligopeptide analog, very similar to the natural substrate of the protease.
One contrary approach that is curently being investigated is to design a therapeutic agent that activates the HIV protease. This very surprising research avenue is based on the demonstration that premature activation of the protease is lethal for the virus. While regulation of the HIV protease is not currently understood, a drug that prematurely activated the protease could be very effective in limiting the amount of viable virions produced by an infected cell.
Combination Therapy
None of the currently available RT or protease inhibitors performs well as a therapy for HIV infection. However, recent advances in medicine can bring an infected individual's viral load to undetectable levels (<200 virions/mL blood). This treatment involves ingesting not just a single drug, but a combination therapy consisting of three chemotherapeutic agents. While certainly not a cure for HIV, this "triple cocktail" drug regimen has been very successful in delaying the onset of AIDS and significantly increasing the quality of life for those infected with HIV. Normally, two RT inhibitors and a protease inhibitor are prescribed, and a strict daily medicinal regimen is required of the patient. Unfortunately, 90% of the HIV-infected individuals live in poor, underdeveloped third-world countries. These people have no access to the expensive combination therapy.
|









