|
|
||||||||||
|
|
|||||||||||
Fat BlockersObesity is a serious health problem. The cost of managing and treating obesity is estimated to represent between two and seven percent of the total health cost in the United States. A common measure of obesity is the body mass index (BMI):
Overweight is defined as BMI>25 while obesity is defined as BMI>30. The prevalence of obesity in the U.S. has increased markedly in the past 25 years. Overall, approximately 50% of the population is either overweight or obese. Hypertension, diabetes, coronary heart disease, gallbladder disease and cancer are only a few of the diseases that are directly or indirectly linked to obesity. Let's see how you rate according to these guidelines (using American non-metric personal statistics): Weight loss has traditionally involved diets or behavioral modification. The problem with both of these approaches is maintaining a desired weight. The elucidation of central and peripheral pathways involved in regulation of body mass has been accompanied by the realization that obesity is a chronic disease and that drugs targeting these pathways can greatly assist the management of obesity. Major targets for anti-obesity drugs include
Development of anti-obesity drugs parallels that seen in the development of drugs to treat hypertension. In the 1950s and 1960s drugs such as reserpine and methyl-dopa were used to treat hypertension. These drugs, because they acted on the central nervous system, had many unwanted side effects. Similarly, many centrally acting appetite suppressants ceased to be used, in part because some were modified amphetamines with addictive properties. "Fen-Phen," a combination of fenfluramine and phentermine, was banned because of reports of heart valve disease.
One approach to the problem of obesity has been to eliminate dietary fat as a source of calories. Olestra, sometimes referred to as "fake fat," is too large to be digested by digestive tract lipases. Dietary fat normally contains many triglycerides (a glycerol backbone to which three fatty acids are esterified.) Pancreatic lipase is the most important of the digestive tract lipases and removes the two terminal fatty acids, leaving a monoglyceride. These products are then transported across the cells lining the small intestine. Olestra consists of a sucrose molecule to which seven fatty acids are attached. This large molecule cannot by hydrolyzed by pancreatic lipase and it passes undigested through the alimentary tract. Side effects associated with Olestra include fecal urgency, anal leakage and a loss of some fat-soluble vitamins. Taking vitamin supplements can alleviate the last side effect. A more complete understanding of the physiology of digestion has led to the development of anti-obesity drugs that act peripherally, that is, at the level of the digestive tract. Compared to esterases that act on water-soluble substrates, intestinal lipases must be able to adsorb to a lipid-water interface. When fat is emulsified or in a micellar form, the activity of lipases is greatly augmented. Three intestinal lipases are of importance: gastric lipase, pancreatic lipase and carboxylester lipase. Carboxylester lipase hydrolyzes vitamin esters and is therefore important in vitamin absorption. Gastric lipase begins the process of fat digestion by forming fatty acids and diglycerides from dietary triglycerides. These fatty acids in turn help activate pancreatic lipase. The bulk of triglyceride digestion is accomplished by pancreatic lipase. In the presence of fatty acids, bile salts and colipase, pancreatic lipase undergoes a dramatic conformational change to expose the active site and allow it to digest triglycerides. Colipase serves to anchor pancreatic lipase to substrate and also to stabilize the lid domain (see figure.) Colipase is secreted by the pancreas as procolipase (an inactive zymogen form) and is activated by trypsin which splits off a pentapeptide from the N-terminus.
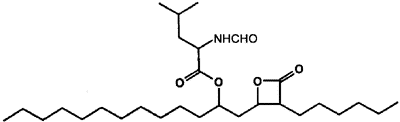
Orlistat (Xenical) is a modified version of lipstatin, a lipase inhibitor produced by the bacterium Streptomyces toxytricini. Orlistat inhibits all three intestinal lipases. Orlistat binds covalently to serine-152 in the active site and effectively prevents access of lipase to other dietary triglycerides. Passage of orlistat across the intestinal epithelium is exceedingly minimal and does not appear to have an effect on lipases other than those in the intestinal tract. Side effects associated with orlistat intake are mainly due to unabsorbed fat passing through the digestive tract and decreased absorption of vitamins D and E. These side effects are considerably reduced after one year. In 1999 the major transporter of fatty acids in the intestine of mice was identified. This transporter is used to transport fatty acids resulting from the breakdown of triglycerides by intestinal lipases. Transporter activity may prove to be a future target for obesity drugs. If so, it might eliminate some of the side effects observed with orlistat and with olestra. |
 |
 |
Copyright 2006, John Wiley & Sons Publishers, Inc. |
 |
 |