 |
|
||||||||||
|
|
|||||||||||
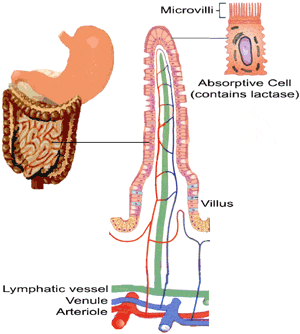
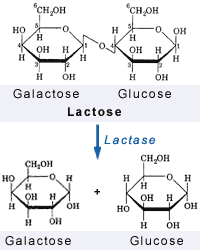
Lactose IntoleranceMilk SugarA feature unique to mammals is the possession of mammary glands devoted to the production of milk for their young. Compared to other mammalian species, human milk has the highest concentration of the disaccharide lactose. The enzyme lactase (EC 3.2.1.23/62) is a membrane bound enzyme located in the brush border or microvilli of the small intestine. Lactase hydrolyzes lactose into its constituent monosaccharides, glucose and galactose, which are then transported across the lining of the small intestine.
It is therefore ironic that as adults, as much as 75% of the world’s human population is intolerant to ingested dietary lactose; common symptoms of intolerance include diarrhea, abdominal pain, flatulence and nausea. An estimated 90% of northern Europeans are, however, lactose tolerant. An intriguing hypothesis proposes that as humans began to populate northern Europe, there was a loss of facial hair and development of a fair complexion as an adaptation to ensure adequate vitamin D production. Furthermore, there was a strong selection for individuals having lactase and the ability to tolerate cow’s milk.
Lactose is unique in that only in milk does it exist as a free form, unattached to other molecules. Lactose is synthesized by lactase synthetase using UDP-galactose and glucose as substrates. Lactose synthetase consists of two subunits: galactosyltransferase plus alpha-lactalbumin. The latter is a modifier subunit that causes galactosyltransferase to transfer galactose to glucose rather than to N-acetylglucosamine moieties found in many glycoproteins. While the catalytic subunit is in abundance during pregnancy, the levels of alpha-lactalbumin are under hormonal control and increase only during the latter part of gestation when prolactin levels increase. Thus, lactose is synthesized for secretion in milk only when needed. Marine mammals such as seals, sea lions and walruses lack alpha-lactalbumin and as a consequence their milk is lacking in lactose and has a very high fat and protein content instead. Feeding cow’s milk (which contains lactose) to a baby walrus in 1933 while it was being transported from Alaska to California resulted in severe diarrhea. Not only do these animals lack lactose in their milk, they also lack the lactase enzyme that is needed for its digestion. The gene coding for lactase is located on chromosome 2. The enzyme is synthesized as a preproprotein having five domains: signal sequence domain, proprotein domain, enzymatic domain, membrane spanning/anchor domain and a hydrophobic C-terminal domain located in the cytoplasm. The signal and proprotein sequences do not appear in the mature enzyme; it has been suggested that proprotein sequence functions as a soluble enzyme with an as yet unknown function.
Examination of the pattern of inheritance of adult lactose intolerance reveals that lactase deficiency is inherited as a recessive trait. The gene for lactase in intolerant and tolerant individuals appears to be normal; some investigators argue that the problem of lactose intolerance is one of post-transcriptional processing while others argue that the levels of mRNA are normal in tolerant and intolerant individuals and that the defect is one of post-translational processing. Whatever the exact cause, intolerant individuals when young have plentiful lactase activity, but as adults lack significant lactase activity in the membranes of the intestinal microvilli. While the direct cause of many metabolic diseases is a mutated enzyme this is apparently not the case in lactose intolerance. There are several methods available to determine if one is lactose intolerant. One method involves ingesting 50 g of lactose; in intolerant subjects this is followed sometime later by abdominal discomfort and an increase in blood glucose of less than 20 mg/dL. More commonly, the breath hydrogen test is utilized. 50 g of lactose are ingested orally; in lactose intolerant individuals lactose is not digested but it is instead fermented by colon bacteria that produce lactic acid and other short chain organic acids plus methane, carbon dioxide and hydrogen gas (H2). H2 is absorbed by the intestinal lining, passed into the circulatory system and exhaled via the lungs where increased levels can be detected. Neither of these methods should be utilized with infants or very young children as the resultant diarrhea can lead to severe dehydration. In these instances, the presence of elevated levels of organic acids in the stool can be detected with a stool acidity test.
In response to ingested food, water and several digestive enzymes are normally added to the contents of the small intestine. It has been known for a long time that water movement out of the small intestine is somehow coupled to the movement of salt and sugars. It is now known that the products of lactose digestion move out of the gut via a co-transporter. The transporter protein binds sodium, glucose (or galactose) and approximately 260 water molecules; sodium moves down its electrochemical gradient and brings glucose or galactose in against a concentration gradient. The failure of water reabsorption in lactose intolerant individuals is responsible in part for the watery diarrhea often observed. The fact that the co-transporter is also a significant water transporter explains the efficacy of including glucose in oral rehydration solutions used in the treatment of cholera. The cholera toxin causes the persistent stimulation of adenylyl cyclase, elevated cAMP levels and increased secretion of chloride ions into the small intestine. The net result is the secretion of copious amounts of water (as much as 2.5 gallons per day) into the intestine. The cholera toxin does not, however, impair the function of the glucose transporter and hence including glucose in the rehydration solution effectively counterbalances the cholera toxin-induced water loss. |
||||||
 |
 |
Copyright 2006, John Wiley & Sons Publishers, Inc. |
 |
 |
 What can one do if one is lactose intolerant? Avoiding dietary lactose is one solution.
Milk made from rice or soy is readily available and does not contain any lactose.
Lactase-containing solutions can be added to milk to digest the lactose or one
can chew lactase tablets; the latter approach is effective for lactose-containing
solid foods.
What can one do if one is lactose intolerant? Avoiding dietary lactose is one solution.
Milk made from rice or soy is readily available and does not contain any lactose.
Lactase-containing solutions can be added to milk to digest the lactose or one
can chew lactase tablets; the latter approach is effective for lactose-containing
solid foods. Because
milk is a major dietary source of calcium and vital for bone growth and maintenance,
it is important that calcium supplements be taken. Some green vegetables such
as broccoli contain significant amounts of calcium.
Because
milk is a major dietary source of calcium and vital for bone growth and maintenance,
it is important that calcium supplements be taken. Some green vegetables such
as broccoli contain significant amounts of calcium.