 |
|
||||||||||
|
|
|||||||||||
Methanol as Fuel:from Bugs to Cars and Cows
|
|
Step 1: |
2CH4 (feed gas) + 3H20 (steam) |
CO + CO2 + 7H2 (synthesis gas) |
The second step is to synthesize methanol from the synthesis gas. The synthesis gas is fed into a reactor vessel under high pressures and temperatures. Carbon dioxide and hydrogen combine (in the presence of a catalyst) to produce methanol:
|
Step 2: |
CO + CO2 + 7H2 |
2CH3OH (methanol) + 2H2 + H2O |
||
|
(catalyst) |
Step 2 is an exothermic (energy-releasing) process, and many methanol plants use the energy released in step 2 to generate the electricity needed for step 1!
 Methanol itself serves as a component in making biodiesel. Biodiesel is any diesel-fuel substitute made from biomass materials like vegetable oil, animal fat, or alcohols. Yes, it’s true, we can make fuel from fat. If you think about it, that’s what fat is for us -- a fuel reserve some of us carry around in greater amounts than we’ll ever need!
Methanol itself serves as a component in making biodiesel. Biodiesel is any diesel-fuel substitute made from biomass materials like vegetable oil, animal fat, or alcohols. Yes, it’s true, we can make fuel from fat. If you think about it, that’s what fat is for us -- a fuel reserve some of us carry around in greater amounts than we’ll ever need!
In Europe, the main source for biodiesel is rapeseed oil, a cousin of canola oil. In the United States, the primary source is soybean oil because the United States produces more soybean oil than all other fats and oils combined. Other potential sources of biodiesel include recycled cooking oil, animal fat, and other oilseed crops.
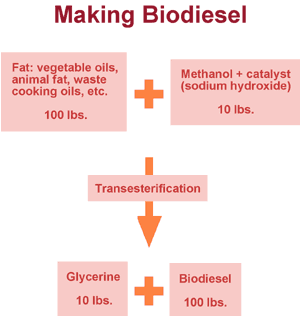
To make fuel from fat, we subject the fat to a process called "transesterification;" in other words, we change it to an ester. The oils used in this process contain triglycerides, which consist of a glycerine molecule with three fatty acids attached. Methanol is mixed with a catalyst -- often sodium hydroxide -- and added to the oil. The resulting mixture -- sodium methoxide -- breaks the triglyceride into glycerine and ester chains. Methyl groups are added where the ester chains once linked to the glycerine. The ester chains are now methyl esters (they would be ethyl esters if the reaction had been carried out with ethanol).
The standard recipe for this process is 100 pounds of fat + 10 pounds of methanol mixed with the catalyst with a yield of about 10 pounds of glycerine and 100 pounds of biodiesel. It’s a very efficient process, and excess methanol can be recovered and used again. Not only does this process use renewable resources, biodiesel fuel burns up to 75 percent cleaner than petroleum-based diesel.
 Biodiesel is not the only use for methanol for transportation purposes. High speed racing vehicles have used methanol for fuel for many years, but a more recent development is the potential for fuel-cell powered vehicles which use a methanol reforming unit to generate hydrogen from methanol. The hydrogen reacts with oxygen in the fuel cell, generating an electrical current. This current may be used to drive a vehicle directly through an electrical traction motor, or the energy can be stored in lithium batteries. The use of methanol as the primary vehicle fuel avoids potential safety problems associated with the storage of compressed hydrogen on board.
Biodiesel is not the only use for methanol for transportation purposes. High speed racing vehicles have used methanol for fuel for many years, but a more recent development is the potential for fuel-cell powered vehicles which use a methanol reforming unit to generate hydrogen from methanol. The hydrogen reacts with oxygen in the fuel cell, generating an electrical current. This current may be used to drive a vehicle directly through an electrical traction motor, or the energy can be stored in lithium batteries. The use of methanol as the primary vehicle fuel avoids potential safety problems associated with the storage of compressed hydrogen on board.
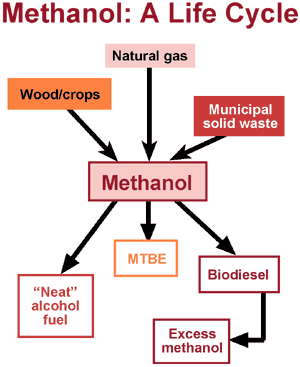
From beginning to end, methanol offers a renewable resource for fuel. We can produce it from many sources -- from natural gas to renewable feedstocks like municipal solid waste or crops. Methanol, in turn, forms a renewable, reusable source for making cleaner-burning biodiesel fuels. Not bad for a little molecule known as the simplest alcohol.
Methane (CH4): from Swamps and Animals
Biologically, methane is produced by a most unusual group of organisms, the methanogens. These organisms are members of the Euryarchaea, a division of the Archaea. The Archaea (formerly called the Archaebacteria) have only relatively recently been recognized as one of the major divisions of living organisms, separate and distinct from the Prokaryotes and the Eukaryotes. One of the unusual features of the Archaea is the type of lipid found in their membranes. The membrane lipids are ether-linked glycerol derivatives, in contrast to the ester-linked lipids found in eukaryotic and prokaryotic membranes. The methanogens are extreme anaerobes. They generate methane gas from carbon dioxide and hydrogen by a complex pathway outlined below.
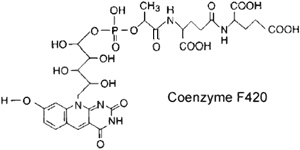
The enzymes of this pathway are extremely oxygen sensitive and employ some unique cofactors. One of these is Coenzyme F420, which is highly fluorescent under ultraviolet light.
 The methanogens really need this complex pathway, since it is the only way they make energy. This means, in turn that they are dependent upon a source of hydrogen, which is supplied by hydrogen secreting anaerobes. In addition to using CO2 as a carbon source, some of the methanogens can utilize other 1-carbon compounds such as formic acid and 2-carbon compounds such as acetate.
The methanogens really need this complex pathway, since it is the only way they make energy. This means, in turn that they are dependent upon a source of hydrogen, which is supplied by hydrogen secreting anaerobes. In addition to using CO2 as a carbon source, some of the methanogens can utilize other 1-carbon compounds such as formic acid and 2-carbon compounds such as acetate.
The methanogens are very common organisms , being found in a wide variety of anaerobic environments. They give rise to swamp gas and are responsible for the natural gas found in oil deposits. Nowadays it is becoming increasingly common for the methane produced in landfills to be trapped and used as fuel for electricity production. Waste- water treatment plants also make use of methanogens. Wastewater treatment is essentially a two stage process. In the first stage, organic materials in the water are utilized as fuel by aerobic organisms and the carbon is converted to biomass. After settling out of the aerobes, anaerobic bacterial digestion converts the biomass to acetate. The acetate is then utilized by the methanogens (especially Methanosarcina species) to generate energy as ATP and to release methane to the atmosphere.
Methane is also an important contributor to greenhouse gases. A surprisingly large percentage of methane emissions come from ruminant animals. For example, it has been estimated that more than 40% of New Zealand’s and 15% of France’s greenhouse gases arise from those country’s sheep and cows. The gases are generated by intestinal microbes, operating anaerobically. Research is underway to determine whether the gas producing organisms might be reduced without affecting the growth of the animals.
 |
 |
Copyright 2006, John Wiley & Sons Publishers, Inc. |
 |
 |
 Methanol: Heightened Interest as a Multi-Use Fuel
Methanol: Heightened Interest as a Multi-Use Fuel