|
|
||||||||||
|
|
|||||||||||
Molecular RecognitionIntroduction
One of the main reasons behind the toxic side effects of a chemotherapeutic agent is nonspecificity. This means that besides the drug's target, the drug also acts on a cell type or enzyme for which it was not intended. For example, many cancer therapies target and destroy rapidly dividing cells (one of the hallmarks of cancer). However, since many healthy cells in the body are also going through cell division, albeit to a smaller degree, the drug effects them as well, causing the side effects of cancer chemotherapy we are familiar with. Another example are the nucleoside analogs (such as AZT) used to treat HIV, all of which target the viral polymerase. Unfortunately, a limiting side effect of these compounds is that they also seem to inhibit the polymerase residing in our mitochondria, inhibiting the mitochondria's ability to replicate in response to an increase of energy requirements of the cell or for cell division.
There are two ways to overcome the problem of side effects. The first is to improve the therapeutic compound so that it binds its intended target more tightly or with higher specificity, so that much lower doses of the drug can be used effectively, well under the levels causing toxicity. The second is to target the drug so that it specifically selects only its intended target. For example, if a chemotherapeutic agent could be delivered only to the location of cancer cells, then its effects on normal cells would be greatly reduced. This necessary selectivity of binding, or molecular recognition, has been achieved using the inherent specificity of proteins and (more recently) nucleic acids. By tethering therapeutic agents to proteins that provide molecular recognition, very selective treatments can be devised. One such example is immunotoxins. Immunotoxins are hybrid molecules combining the targeting selectivity of antibodies with the cell killing abilities of a protein toxin. Linking toxins to antibodies is a promising approach for the treatment of cancer and AIDS. AIDSAIDS (Acquired Immunodeficiency Syndrome) is a devastating disease in which the immune system of an individual infected with the Human Immunodeficiency Virus (HIV) is severely compromised due to the destruction of a population of white blood cells termed T-helper lymphocytes. As of December, 1999, approximately 400,000 Americans were either infected with HIV or had the overt symptoms of AIDS. The incidence of AIDS is on the rise in sub-Saharan Africa and Southeast Asia where approximately 14 million individuals are infected. Much effort has been expended to develop a vaccine for AIDS and to develop suitable drug treatments to cure the disease or at least keep in check. Both of these approaches must contend with the inherently high mutation rate of HIV, which contributes to the development of drug resistance and can render vaccines useless. AIDS investigators and others want to use immunotoxins to destroy HIV-infected cells. Such an approach might avoid the problems of drug resistance by targeting proteins that are conserved among different strains of HIV. Toxins
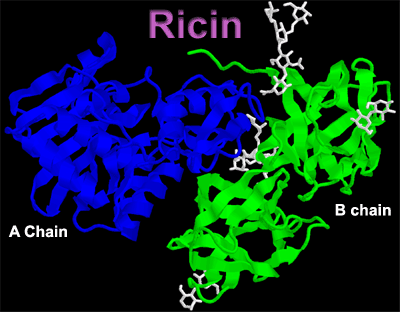
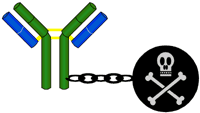
Bacterial and plant toxins are some of the most toxic substances known. On the one hand they can be assassins and on the other hand they can be saviors. Ingestion of ricin, an exotoxin found in seeds of the castor bean plant (Ricin communis), is often lethal. One milligram of ricin is sufficient to kill an adult human. In contrast, ricin and other toxins are being studied intensively for their ability to combat diseases. Ricin, Pseudomonas exotoxin and diphtheria toxin all disrupt cellular protein synthesis and are being actively investigated as therapeutic agents for AIDS and cancer. In order for the immunotoxin approach to be effective, the toxin of choice must be extremely cytotoxic. Pseudomonas and diphtheria toxins are enzymes and attach an ADP-ribose group to the elongation factor 2 (EF2), thereby halting protein synthesis. Because inactivation is a catalytic process, only minute amounts of toxin are necessary. Ricin is synthesized in the endosperm of maturing Ricin communis seeds; once the seed germinates, ricin is destroyed. This is clearly of advantage to the plant as it limits seed consumption by birds and other small animals. All parts of the plant are toxic, however. Castor oil, a purgative and the scourge of young children years ago, is made from the seed oil; heat treatment and further purification eliminate toxicity. Functional ricin has two chains, A and B, linked by a single disulfide bond. The B chain binds to galactose residues of cell membrane glycoproteins and glycolipids. Subsequent endocytosis acts to internalize the entire ricin molecule. The A chain is enzymatically active and depurinates an adenine in the 28s subunit of ribosomal RNA. This irreversibly disrupts protein synthesis. Why doesn’t ricin disrupt protein synthesis in the maturing seed? Ricin is synthesized as a single large preproprotein, that is, it has a signal sequence targeting it to the endoplasmic reticulum in addition to both A and B chain amino acid sequences. After the signal sequence is cleaved off, a disulfide bridge between the A and B chains is catalyzed and the proricin protein is transported from the Golgi apparatus to vacuoles within the seed. Until the A and B chains are enzymatically separated in the vacuole, ricin is inactive. In this way the seed avoids disrupting its own protein synthesis. Because the B chain binds to cell surface glycoproteins and glycolipids in a non-specific fashion, how can ricin be used to disrupt protein synthesis in only certain cells? To accomplish this, the A chain alone is linked to antibodies that recognize only certain cell types. AntibodiesAntibodies are "Y"-shaped proteins produced by white blood cells known as B-lymphocytes. A specific B-lymphocyte produces only one kind of antibody and that antibody can recognize only one particular part (an epitope or antigenic determinant) on an antigen. The two "arms" of the Y are the antigen-binding sites. Originally, antibodies were produced by injecting an animal with a particular antigen of interest. Since antigens commonly have many epitopes that are recognized by lymphocytes, a very heterogeneous mixture of antibodies was the result. If one ran out of antibody, one had to repeat the procedure. There existed a real need for producing, in vitro, significant quantities of antibodies that recognized only a single epitope. Kohler and Milstein developed a procedure for producing monoclonal antibodies for which they received the Nobel Prize in 1984. The production of monoclonal antibodies involves injecting mice with antigen, removing the spleen and isolating B-lymphocytes. The B-lymphocytes (which cannot be propagated in vitro) are fused with myeloma cells (immortal cells) to produce hybridomas. With the use of appropriate techniques, the hybridoma cells can be cloned so that one has a population of cells producing only one particular antibody. The clones are screened for those that produce antibody of the desired specificity. In this way essentially unlimited quantities of antibody recognizing only one epitope can be produced. ImmunotoxinsUsing what are termed heterobifunctional reagents, the A chain of ricin can be linked to a monoclonal antibody.
There are two difficult questions that need to be addressed when designing an immunotoxin. First, against what epitope should the antibody be directed? The answer derives from the observation that cells routinely display on their surface fragments of normal cell proteins processed within the cell. The protein fragments are bound to class I MHC (Major Histocompatibility Complex) proteins. Similarly, fragments of viral (and bacterial) proteins are displayed bound to class I MHC proteins. There are several proteins associated with HIV that are suitable antigens against which monoclonal antibodies can be prepared: gp120 and gp41, two envelope glycoproteins. Human T-helper lymphocytes infected with HIV display these proteins on their cell membranes. (Gp120 binds to CD4, one of the cell membrane proteins on T-helper cells that affords HIV entry into the cell; gp41 mediates the necessary fusion of the HIV envelope and the cell membrane.) Second, how can the antibody-ricin conjugate be transferred from the extracellular environment to the interior of the cell one wishes to eliminate? Endocytosis is a common method by which a variety of cell membrane proteins and associated macromolecules can be internalized. In this case, a monoclonal antibody to which the ricin A chain is bound recognizes gp120 or gp41 on the surface of an HIV-infected T-helper lymphocyte. The antibody-ricin complex is brought into the interior of the cell in a membrane-bound vesicle. At some point the complex is released into the cytoplasm of the cell and the A chain then acts to disrupt protein synthesis.
In humans, using mouse monoclonal antibodies ultimately results in the rapid clearance of the antibodies (and attached ricin A chains) from the body. One solution has been to genetically engineer chimeric antibodies in which the antigen-binding site is of mouse origin and the part of the antibody that identifies it as being "human" as opposed to "mouse" is identical to that of human antibodies; such antibodies are referred to as humanized. AIDS patients and cancer patients being given chemotherapeutic drugs are often immunocompromised, that is, their immune systems are not functioning properly; these individuals will clear foreign antibodies from their bodies more slowly. There is great interest in using antibodies coupled to ricin as a tool to attack HIV-infected cells. Antibody-ricin A chain complexes have been utilized in the treatment of some leukemias in which T-lymphocytes are overproduced. In these cases, a sample of the bone marrow from the leukemia patient is removed from the body. This sample will, of course, contain both normal and cancerous T-lymphocytes. The antibody-ricin complex is used to destroy any T-lymphocytes. After destroying the remaining bone marrow in the patient, the bone marrow sample minus any cancerous cells is reinfused into the patient. Antibody-ricin technology works well against targets such as blood cells because they are readily accessible. Unfortunately, solid tumors have been fairly refractile to this technology. One particularly interesting approach to drug design is to combine the concept of an immunotoxin with the enzymatic activities of an abzyme, or catalytic antibody (see the catalytic antibodies cutting edge article). This hybrid antibody, half targeting moiety, half enzymatic moiety, has been developed for the treatment of cancer. |
 |
 |
Copyright 2006, John Wiley & Sons Publishers, Inc. |
 |
 |
 In the development of a drug treatment (chemotherapy) for a disease, any undesirable side effects are of utmost concern. Often, compounds that function extremely well in benchtop assays, cell culture drug screens, and even in animal testing are found to be severely limited in their use in humans due to their toxic side effects. In fact, toxicity is generally the dose-limiting factor in designing a particular drug regimen for patients.
In the development of a drug treatment (chemotherapy) for a disease, any undesirable side effects are of utmost concern. Often, compounds that function extremely well in benchtop assays, cell culture drug screens, and even in animal testing are found to be severely limited in their use in humans due to their toxic side effects. In fact, toxicity is generally the dose-limiting factor in designing a particular drug regimen for patients.