|
||||||||||||
|
|
||||||||||||
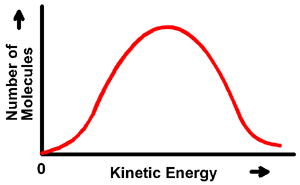
Elementary KineticsWhat Affects Reaction RatesTo understand what affects reaction rates, first we have to know a little bit about collision theory. This sounds ominous, but the whole can be summed up as follows: "You can’t react if you don’t collide."In other words, for chemical reactions to occur, the molecules involved actually have to bump into each other. To help make collision theory and its consequences a bit more clear, we can use bumper cars as an analogy. Think of each of the molecules in a reaction vessel as a bumper car. If you were watching a bumper car ride from the sidelines, you would notice that some bumper cars are moving very slowly, others are going really fast, but most are going at a sort of medium speed. In the same way, in any given mixture of molecules, some of the molecules have high energy, some low, and a lot in between. This idea is often shown as a energy distribution graph that looks like a bell curve:
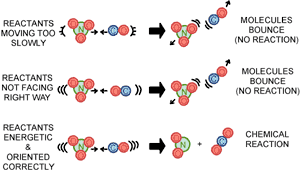
To understand what this distribution of energy idea has to do with how a chemical reaction takes place, let us go back to the bumper car analogy. The best part of the bumper car ride is to bump into another car head-on at a nice fast speed, so that you get a really good "bump" out of your seat. If you aren’t going fast enough when you hit another car, you don’t get a very energetic bump. In a chemical reaction, if a molecule collides with another at a slow speed, no chemical bonds are broken and the molecules just bounce off each other. For a reaction to occur, the collision needs to be forceful enough to break the existing chemical bonds of the reactants. What’s more, the reacting molecules have to bump into each in the proper orientation. They have to be facing the right way so that the new chemical bonds can be formed. If they aren’t, the reactant bonds will break, but the right chemical bonds to form the product won’t be able to connect. It’s a lot like being on that bumper car ride. If you get hit from the side, you just slide sideways on the seat rather than getting bounced up. Let’s look at a real reaction: NO3(gas) + CO(gas)
Temperature and the concentration of reactants are both factors that can affect how many molecules can run into each other with the correct energy and orientation for a chemical reaction to take place. Let’s see why this is the case:
Example: In chemistry lab, you are told to figure out the rate of the following chemical reaction (you can detect how much I3- is formed because it forms a blue color with starch): 3 I-(aq) + S2O82-(aq) You mix the reactants, and two minutes later you measure how much blue color has been formed. You then calculate the rate of I3- formation per minute by the formula: rate = (total product formed) / (time elapsed) Since your lab partner isn’t there yet, you leave the reaction going on your benchtop. Fifteen minutes later, your lab partner finally shows up and decides to check your work. He measures how much blue color has been formed, and finds out from you that the reaction has been going for 20 min. He then calculates the rate of I3- formation from his data. When you compare answers, his rate is much lower than yours. You check each other’s math, no problems there. You used the same formula to calculate the reaction rate, why aren’t your answers the same? After thinking about it for a while, you remember from learning about kinetics that one of the things that can affect the reaction rate is the concentration of the reactants. You measured the initial rate, that is, the rate of the reaction when the substrate concentration had not yet been significantly depleted. (This is the fastest that the reaction will go). As the reaction proceeded, substrate was used up and the reaction slowed down. Thus, when your lab partner measured the reaction rate over a much longer time period, his value came out much lower because the reaction had slowed down. |
 |
Copyright 2006, John Wiley & Sons Publishers, Inc. |
 |