|
||||||||||||
|
|
||||||||||||
Elementary Kinetics
|
 |
|
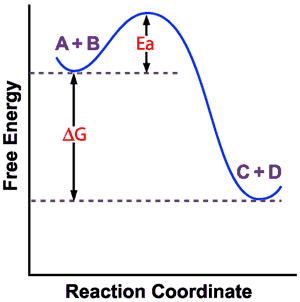
Notice how in converting from reactants to products, the molecules need to temporarily become much more energetic in order to break and remake chemical bonds. This energy "barrier" is referred to as the "energy of activation" (Ea). If the reactants don’t have enough kinetic energy to get over the "hump," the reaction won’t proceed, even if it’s thermodynamically favorable.
 Going back to our example of the skier, if she is to get to the bottom of the ski run, she will need to have enough speed coming into that hill to get up and over the final hump to the finish line.
Going back to our example of the skier, if she is to get to the bottom of the ski run, she will need to have enough speed coming into that hill to get up and over the final hump to the finish line.
We remember from collision theory that the individual reactant molecules have a distribution of energy levels. Some have a low amount of kinetic energy (are moving slowly), whereas others have a high amount of kinetic energy (are moving more quickly). Only those molecules that have enough kinetic energy to make it over the Ea "hump" will react to become products.
From this example, it makes sense that reactions with a lower Ea "hump" will proceed more quickly, because more molecules are likely to have enough energy to overcome that energy barrier. Likewise, reactions with a very tall Ea "hump" will proceed very slowly, because it is unlikely that many molecules will be energetic enough to make it up and over that hill. Thus, for a reaction to be kinetically favorable, it needs to have a low energy of activation.
An important example of a reaction that is thermodynamically favorable, but kinetically unfavorable is:
ATP + H2O ![]() ADP + Pi DG = -30.5 kJ/mol
ADP + Pi DG = -30.5 kJ/mol
With its negative DG value, this reaction for the breakdown of ATP is thermodynamically favorable. Lots of energy is released when ATP is hydrolyzed. However, ATP is kinetically stable because the hydrolysis reaction has a high energy of activation. In other words, for this reaction to happen, ATP has to overcome a tall energy barrier. These properties make ATP the perfect "energy currency" for use in our bodies. Useful energy for biochemical reactions is safely trapped in the ATP molecule until released by an enzyme that needs the energy to "pay" for an energy-requiring chemical reaction. The enzyme grabs an ATP molecule and helps it over the Ea "hump," and then channels that energy from the ATP hydrolysis to the chemical reaction that needs it.
 |
Copyright 2006, John Wiley & Sons Publishers, Inc. |
 |
 Kinetic vs. Thermodynamic Stability
Kinetic vs. Thermodynamic Stability