|
||||||||||||
|
|
||||||||||||
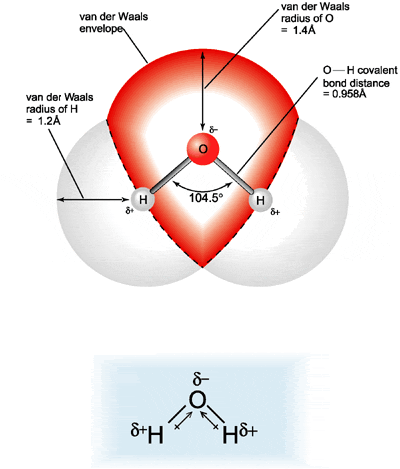
Water, pH, and Non-Covalent BondingWaterWater is essential for life. It covers 2/3 of the earth's surface and every living thing is dependent upon it. The human body is comprised of over 70% water, and it is a major component of many bodily fluids including blood, urine, and saliva. What accounts for the ubiquitous use of water in living systems? If we take a step back and consider the structure of water and compare it to another substance, methane, we can understand the unique properties water possesses that make it well suited for biological systems. Water (H2O) is made up of 2 hydrogen atoms and one oxygen atom, with a total atomic weight of 18 daltons. The structure of the electrons surrounding water is tetrahedral, resembling a pyramid. For comparison, Methane (CH4) is made up of one carbon and 4 hydrogens. Note that methane is similar to water in that it weighs 16 daltons and also has a tetrahedral structure, yet has very different physical properties.
All the vertices on the methane that form the points of a pyramid are occupied by a hydrogen. However, only two vertices in water are occupied with hydrogens. The other two vertices are each occupied by a lone pair of electrons. This fact causes the water molecule to have a bent molecular shape. Oxygen is highly electrophilic (electron loving). This means that even though the oxygen in water is bound to each of the hydrogens by a covalent bond (sharing a pair of electrons), the oxygen "pulls" the shared electrons closer to itself. This unequal sharing of the electrons in the O-H bond in water causes the hydrogens to have a partial positive charge (positive dipole), and the oxygen has a partial negative charge (negative dipole). Water is called a polar molecule because it has a positive side and a negative side, called a dipole moment. In contrast, the carbon in methane shares electrons equally with the 4 hydrogens. Methane's does not have a dipole moment, and in contrast with water is non-polar.
Water molecules, then, have both a partial positive and a partial negative charge. Since opposites attract, this means that water molecules are attracted to each other, like the positive and negative ends of a magnet. Because of these attractive forces, water molecules are in close proximity to one another, making water very dense. Methane molecules do not have a dipole attraction for one another, and therefore and are spaced farther apart. Therefore, despite its similar size and mass to water, methane is much less dense than water. This is the reason that under room temperature situations, water exists as a liquid while methane is a gas. We know that water can be converted to its gaseous phase, steam, but only by applying a lot of energy in the form of heat to disrupt the large attraction of the water molecules for one another.
|
|
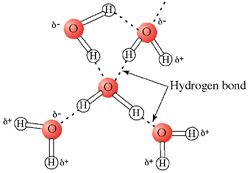
Each hydrogen bond has an average energy of 20 kJ/mol. This is much less than an O-H covalent bond, which is 460 kJ/mol. Even though an individual hydrogen bond is relatively weak, the large number of hydrogen bonds that exist in water which pull the molecules together give water its special density and phase transition properties. This is much like the story of Gulliver's Travels, when Gulliver awoke on the island of Lilliput to find himself tied down with many tiny strings. Each string was individually weak, but together they were enough to bind Gulliver.
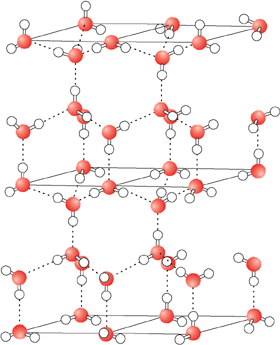 On average, a liquid water molecule will have 3 out of 4 possible hydrogen bonds. This is because the molecules in liquid are in constant motion. In ice, water will form all 4 possible hydrogen bonds because the molecules in a solid are essentially locked in place. Water molecules are further apart from one another in ice than they are in the liquid state. Ice is therefore not as dense as liquid water. This is why icebergs float in the ocean and why ice floats in a beverage. This contrasts greatly from the normal relationship between a compound's solid and liquid forms, with the solid form usually being of a higher density. While the unusual phase density properties of water might not seem too important in biological reactions, it is very important for life on earth. If ice did not float, then in very cold bodies of water ice would sink, removing its insulating effects from the water's surface. Lakes, rivers, and major portions of the oceans thus would freeze completely from the bottom up. The world would be a much different place, and much less suited for life.
On average, a liquid water molecule will have 3 out of 4 possible hydrogen bonds. This is because the molecules in liquid are in constant motion. In ice, water will form all 4 possible hydrogen bonds because the molecules in a solid are essentially locked in place. Water molecules are further apart from one another in ice than they are in the liquid state. Ice is therefore not as dense as liquid water. This is why icebergs float in the ocean and why ice floats in a beverage. This contrasts greatly from the normal relationship between a compound's solid and liquid forms, with the solid form usually being of a higher density. While the unusual phase density properties of water might not seem too important in biological reactions, it is very important for life on earth. If ice did not float, then in very cold bodies of water ice would sink, removing its insulating effects from the water's surface. Lakes, rivers, and major portions of the oceans thus would freeze completely from the bottom up. The world would be a much different place, and much less suited for life.
Water is a good solvent
The polar nature of water, with its partial positive and partial negative dipole, allows it to dissolve charged molecules (ions) easily. Water is thus an excellent solvent for charged compounds. The positive side of water surrounds negatively charged molecules, and the negatively charged side of water surrounds positively charged molecules.
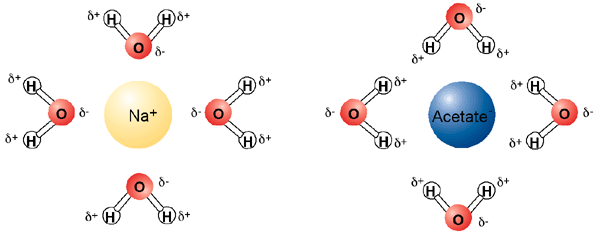
In this way, water makes "solvation shells" around ions. Water can also readily dissolve other polar molecules, even if they are not positively or negatively charged. The solvent properties of water allow for dissolved metals and buffering systems that are very important for the workhorses of life, enzymes. However, the saying "oil and water don't mix" is true--water cannot dissolve oil. This is because oily substances are non-polar. Non-polar substances (which lack dipoles) are also called hydrophobic (water fearing). Hydrophobic substances gather together to exclude water as best they can. This is why you see oil droplets in water. This is also important for the stability and structure of enzymes.
 |
Copyright 2006, John Wiley & Sons Publishers, Inc. |
 |
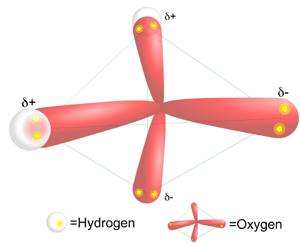
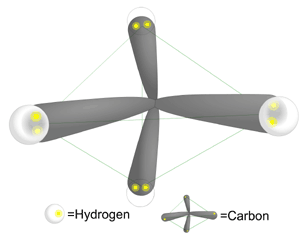
 Hydrogen Bonding
Hydrogen Bonding