Conclusion
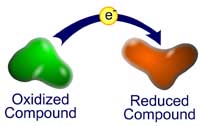 A redox reaction, also known as an oxidation-reduction reaction, is a type of chemical reaction where one of the reactants is oxidized and one of the reactants is reduced. Oxidation of a compound can be defined in several ways, one of which is that it is the gain of bonds to oxygen, another of which is that it is the loss of electrons. Similarly, two useful definitions of reduction, (the opposite of oxidation), are the gain of hydrogen or the gain of electrons.
A redox reaction, also known as an oxidation-reduction reaction, is a type of chemical reaction where one of the reactants is oxidized and one of the reactants is reduced. Oxidation of a compound can be defined in several ways, one of which is that it is the gain of bonds to oxygen, another of which is that it is the loss of electrons. Similarly, two useful definitions of reduction, (the opposite of oxidation), are the gain of hydrogen or the gain of electrons.
The mnemonic OIL RIG can help you remember these definitions: Oxidation Is Loss (of electrons), Reduction Is Gain (of electrons).
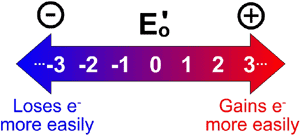 Oxidation and reduction reactions always occur together, because the electrons that are donated from one compound, must be received by another compound. This is why redox reactions are said to be the product of two half reactions, the oxidation half reaction and the reduction half reaction. Each half reaction has a measurable reduction potential E0, which is a measure in volts of how easily the compound is reduced (how easily it gains electrons). Remember, the reduction potential is how much a species "wants" to get reduced, and the higher the number, the greater the potential.
Oxidation and reduction reactions always occur together, because the electrons that are donated from one compound, must be received by another compound. This is why redox reactions are said to be the product of two half reactions, the oxidation half reaction and the reduction half reaction. Each half reaction has a measurable reduction potential E0, which is a measure in volts of how easily the compound is reduced (how easily it gains electrons). Remember, the reduction potential is how much a species "wants" to get reduced, and the higher the number, the greater the potential.
Redox reactions play an important part in our lives. Combustion reactions that generate heat and electricity, such as the burning of natural gas, oil, gasoline or wood, are redox reactions, and in our bodies, redox reactions are needed to generate ATP to power our metabolism and our muscles.


 A redox reaction, also known as an oxidation-reduction reaction, is a type of chemical reaction where one of the reactants is oxidized and one of the reactants is reduced. Oxidation of a compound can be defined in several ways, one of which is that it is the gain of bonds to oxygen, another of which is that it is the loss of electrons. Similarly, two useful definitions of reduction, (the opposite of oxidation), are the gain of hydrogen or the gain of electrons.
A redox reaction, also known as an oxidation-reduction reaction, is a type of chemical reaction where one of the reactants is oxidized and one of the reactants is reduced. Oxidation of a compound can be defined in several ways, one of which is that it is the gain of bonds to oxygen, another of which is that it is the loss of electrons. Similarly, two useful definitions of reduction, (the opposite of oxidation), are the gain of hydrogen or the gain of electrons. Oxidation and reduction reactions always occur together, because the electrons that are donated from one compound, must be received by another compound. This is why redox reactions are said to be the product of two half reactions, the oxidation half reaction and the reduction half reaction. Each half reaction has a measurable reduction potential E0, which is a measure in volts of how easily the compound is reduced (how easily it gains electrons). Remember, the reduction potential is how much a species "wants" to get reduced, and the higher the number, the greater the potential.
Oxidation and reduction reactions always occur together, because the electrons that are donated from one compound, must be received by another compound. This is why redox reactions are said to be the product of two half reactions, the oxidation half reaction and the reduction half reaction. Each half reaction has a measurable reduction potential E0, which is a measure in volts of how easily the compound is reduced (how easily it gains electrons). Remember, the reduction potential is how much a species "wants" to get reduced, and the higher the number, the greater the potential.