|
||||||||||||
|
|
||||||||||||
Redox ReactionsReduction Potentials
We just discussed in the last section how a redox reaction is really two reactions that happen together, an oxidation and a reduction. It makes sense that they occur together if we think about how oxidations lose electrons while reductions gain electrons (remember OIL RIG). The two reactions must be coupled because you can’t have an electron donor without an electron recipient. Because of this, these two reactions (oxidation and reduction) are considered to be two halves of a whole. In other words, a redox reaction consists of an oxidation half reaction and a reduction half reaction. Let’s return to the OIL RIG definition of redox reactions for a moment. It can tell us which compounds are oxidized or reduced, depending on whether electrons are gained or lost. But which atoms gain electrons, and which ones lose them? Remember that electrons are tiny charged particles that orbit the nucleus of an atom at dizzying speeds. Depending on how many electrons are orbiting the nucleus, and in which orbitals (at what distance from the nucleus), some atoms hold on to some of their electrons very loosely, and have a higher tendency to give them away. Other atoms hold onto their electrons very tightly, and are in fact able to attract nearby electrons into their orbitals. The potential, or tendency, to gain electrons, is the reduction potential. The reduction potential is the tendency of a compound to be reduced. The reduction potential is experimentally determined for the oxidation and reduction half reactions, and is called E0. To remember the relationship of E0 and oxidation and reduction, consider the reduction potential as how much a compound wants to get reduced (it has a lot of potential for reduction). Remember, reduction is a gain of electrons (OIL RIG), so the larger (more positive) the E0, the more likely the compound in the half reaction is to gain electrons (be reduced).
The E0 values can be looked up in tables, and are always given for reduction half–reactions (reactions gaining electrons). For example: S + 2H+ + e-
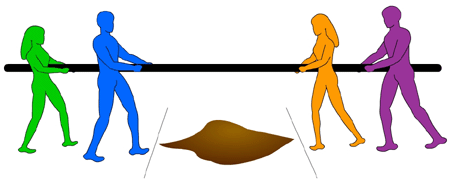
If a compound is less likely to be reduced, it is more likely to be oxidized. Thus, in a redox reaction, when two half-reactions are coupled together, the half-reaction (with the higher E0) will be the reduction reaction, while the half-reaction with a smaller (or more negative) E0 will then be forced backwards and act as the oxidation reaction. A knowledge of the half-reaction reduction potentials can therefore tell you whether compounds are more likely to be oxidized or reduced. It’s like a tug-of-war. The strongest team always pulls the weaker team into the mud pit. In the same way, the compound with the larger reduction potential pulls the electrons over to its side.
Finally, the tendency of the whole reaction to proceed can be calculated from reduction potentials of half reactions, which have been determined experimentally, using the formula: DE0 = (E0 from reaction where e- are gained) - (E0 from reaction where e- are released) A positive DE0 tells you that the reaction will proceed in the direction it is written. Let’s do an example to make all these rules seem a little more clear. In a reaction that is needed for ATP generation in our bodies, cytochrome C molecules are oxidized in a redox reaction where oxygen that we breathe is reduced: 4 cytochrome c2+ + 4 H+ + O2 The two half-reactions are:
Which of the half reactions is more likely to gain electrons? What is the overall DE0 of the redox reaction? Notice how the half reactions are both written as reductions. The positive (larger) value for E0 means that the half reaction will insist on gaining electrons, and act as the reduction. Reaction (2) has the higher E0, and is thus more likely to gain electrons. Because reaction (1) is by comparison less likely to gain electrons, reaction (1) will be forced backwards, becoming an oxidation reaction, by reaction (2). Thus, in order to "add" the two half reactions together to get the whole reaction, we will have to reverse half reaction (1): cytochrome c2+ Now let’s add the two half reactions to get the whole redox reaction. Notice that we will have to multiply the above reaction [reverse of (1)] by 4, and reaction (2) by 2, to get the final reaction. However, the E0 values are not multiplied! This is because the DE0 value is independent of the numbers of electrons that are in the half reactions.
|
 |
Copyright 2006, John Wiley & Sons Publishers, Inc. |
 |
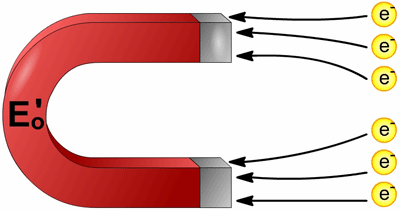 Think of it as being like a large magnet. A large magnet attracts nails more strongly, and holds them more tightly, than a small magnet. Similarly, a compound with a large (positive) reduction potential attracts electrons more strongly than a small (or even a negative) reduction potential.
Think of it as being like a large magnet. A large magnet attracts nails more strongly, and holds them more tightly, than a small magnet. Similarly, a compound with a large (positive) reduction potential attracts electrons more strongly than a small (or even a negative) reduction potential.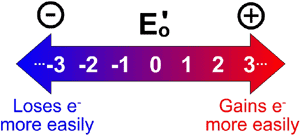 We know that a redox reaction consists of both a reduction and an oxidation. Shouldn’t there be such a thing as an "oxidation potential," too? As it turns out, you can tell how likely a compound is to be oxidized from the reduction potential. Since, as mentioned above, a high (large positive) reduction potential means a high tendency to be reduced, it must follow that a lower reduction potential must mean that a compound is less likely to be reduced.
We know that a redox reaction consists of both a reduction and an oxidation. Shouldn’t there be such a thing as an "oxidation potential," too? As it turns out, you can tell how likely a compound is to be oxidized from the reduction potential. Since, as mentioned above, a high (large positive) reduction potential means a high tendency to be reduced, it must follow that a lower reduction potential must mean that a compound is less likely to be reduced.