|
||||||||||||
|
|
||||||||||||
Thermodynamics ReviewCoupled reactions in biochemical processes/pathwaysEven though the universe is continually becoming more disordered (following the second law of thermodynamics), not everything is utter chaos. For instance, we know that our bodies are highly organized. Each of our different organs and tissues performs unique functions, and even at the cellular level molecules are partitioned into organelles and compartments. Because they possess such highly organized structure, living organisms are said to have a relatively low amount of entropy. To sustain life, organisms need to build up a complex body from more basic building blocks. Complex proteins are made from long chains of amino acids, precisely and intricately folded. DNA is similarly comprised of long chains of nucleic acids. We know from the second law of thermodynamics that the reactions that create these molecules of life are certainly not spontaneous; energy input is required to make them go. Organisms do not just magically assemble themselves. But how do living things deliver the needed energy to such body-building chemical reactions?
Thermodynamics lets us predict that the reaction will proceed if the overall DG of the two reactions is negative. When two reactions are coupled, the overall DG is the sum of the DGs of the component reactions.
For example, in the biochemical pathway that breaks down glucose for energy, two enzymes work one after the other to create a high-energy ATP molecule: Enzyme 1+ cofactor: Enzyme 2
Example: The first step in breaking down glucose for energy consists of adding a phosphoryl group onto a glucose molecule; a reaction catalyzed by the enzyme hexokinase:
We would like to know what the free energy for this reaction is. However, we only know that:
and
So using what we know about coupled reactions, what is the overall DG, and Keq of reaction (1)? Solution: To answer these questions, we can add the reactions together (note how the H2O terms cancel out):
To find the DG of the overall reaction, simply add the DGs of the component reactions together: DG1 = DG2 + DG3 To find the Keq of the overall reaction, you need to multiply the Keqs of the reactions together: Keq1 = [Keq2][Keq3] |
 |
Copyright 2006, John Wiley & Sons Publishers, Inc. |
 |
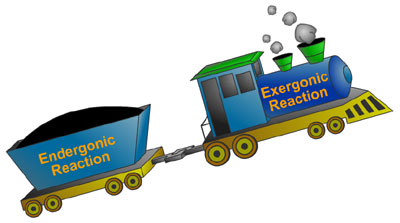 We have already discussed that some reactions are exergonic (they release energy that might be useful for work), while others are endergonic (they need energy to make them go). To get the energy to those endergonic reactions, they are paired up with energy-releasing exergonic reactions. Like a locomotive that gets the train car over the hill, an exergonic reaction can "pull" an endergonic reaction along to its destination (products). The reactions can be hooked together, or coupled, via a common intermediate.
We have already discussed that some reactions are exergonic (they release energy that might be useful for work), while others are endergonic (they need energy to make them go). To get the energy to those endergonic reactions, they are paired up with energy-releasing exergonic reactions. Like a locomotive that gets the train car over the hill, an exergonic reaction can "pull" an endergonic reaction along to its destination (products). The reactions can be hooked together, or coupled, via a common intermediate.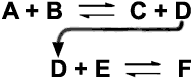
 The sum of the free energy for these two reactions gives the overall DG for this process (DG = -16.7 kJ/mol), which is very thermodynamically favorable. Another way in which reactions can be coupled is by breaking down a high energy compound with an enzyme, trapping that energy and using it to drive an endergonic reaction. You can think of energy as chemical currency used to conduct business in the cell. Taking this energy-as-money analogy a bit further, the coupling of the reactions is like what Robin Hood did in the Sherwood forest; stole from the rich and gave to the poor. Coupling reactions is a way to take the energy from the "rich" and bring it to the "poor" that really need it.
The sum of the free energy for these two reactions gives the overall DG for this process (DG = -16.7 kJ/mol), which is very thermodynamically favorable. Another way in which reactions can be coupled is by breaking down a high energy compound with an enzyme, trapping that energy and using it to drive an endergonic reaction. You can think of energy as chemical currency used to conduct business in the cell. Taking this energy-as-money analogy a bit further, the coupling of the reactions is like what Robin Hood did in the Sherwood forest; stole from the rich and gave to the poor. Coupling reactions is a way to take the energy from the "rich" and bring it to the "poor" that really need it.