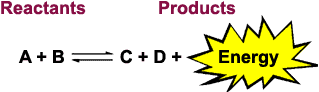|
||||||||||||
|
|
||||||||||||
Thermodynamics Review
|
Exergonic Reaction |
Endergonic Reaction |
|
Spontaneous |
Not Spontaneous |
|
Release Free Energy ( -DG) |
Consume Gibbs Free Energy (+DG) |
|
Products have less energy than reactants |
Products have more energy than reactants |
For example, here is the reaction when the high energy compound ATP is hydrolyzed to release an inorganic phosphate molecule and energy:
ATP + H2O ![]() ADP + Pi DG = -30.5 kJ/mol
ADP + Pi DG = -30.5 kJ/mol
Note that when the reaction releases energy, DG is negative!
When the reaction is written in reverse, the sign of DG changes:
ADP + Pi ![]() ATP + H2O DG = +30.5 kJ/mol
ATP + H2O DG = +30.5 kJ/mol
 |
Copyright 2006, John Wiley & Sons Publishers, Inc. |
 |
 Gibbs Free Energy
Gibbs Free Energy