Water, pH, and Non-Covalent Bonding
pH questions
Below are a few sample questions to elucidate the concepts discussed in the book.
A simple pH problem
What is the pH 0.1 M HCl?
Answer:
The pH of a solution is the negative logarithm of the concentration of H+ ions. In this case, HCl is a very strong acid. HCl completely dissociates to H+ and Cl-.
HCl 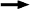 H++ Cl- H++ Cl-
The concentration of HCl is 0.1 M, if all of it dissociates to H+, then
[H+] = 0.1
-log(0.1) = 1.26
pH = 1.26
Remember that a pH of 7 is neutral, and that the pH scale is logarithmic, meaning each pH unit represents a factor of 10. This HCl solution has a difference of 5.74 pH units from water (7 - 1.26). Taking the inverse log of 5.74 means that this HCl solution is more than 500,000 times as acidic as water!

pH of a weak acid solution
What is the pH of a 0.05 M solution of acetic acid? (The Ka of acetic acid = 1.8 x 10-5)
Answer:
Determining the pH of a weak acid solution is more complicated than for a strong acid. This is because a weak acid does not completely dissociate.
CH3COOH  H+ + CH3COO- H+ + CH3COO-
To simplify matters, it is helpful to construct a grid:
| Reaction |
CH3COOH |
H+ |
CH3COO- |
| Initial |
0.05 M
|
0 M
|
0 M
|
| Change |
(-x) M
|
+ x M
|
+x M
|
| Equilibrium |
(0.05-x) M
|
x M
|
x M
|
To determine the [H+], we must employ the Ka of acetic acid, which is 1.8 x 10-5.
|
Ka =
|
[H+][CH3COO-]
[CH3COOH]
|
= 1.8 x 10-5 |
Substituting the equilibrium values in the table above, we get:
|
1.8 x 10 -5 =
|
(x M)(x M)
(0.05 - x) M
|
Since acetic acid is a weak acid, we can assume that very little of it will dissociate. This simplifies the above equation to:
|
1.8 x 10 -5 =
|
(x M)(x M)
0.05 M
|
This can be easily solved:
9 x 10-7 M = x2 M
x = 9.5 x 10-4 M
x = 0.00095 M
Now that we have the [H+], we can solve for pH:
pH = -log[0.00095]
pH = 3

Buffer Problem
Question 1: What is the ratio of [lactate]/[lactic acid] at pH = 5? (The pKa of lactic acid = 3.85)
Answer:
To solve this problem, we need to utilize the Henderson-Hasselbalch equation:
|
pH =
|
pKa + log
|
[H+][A-]
[HA]
|
Table 3.3 in the book lists the pKa of lactic acid as 3.85. Now just substitute into the equation:
|
5 =
|
3.85 + log
|
[lactate]
[lactic acid] |
|
1.15 =
|
log
|
[lactate]
[lactic acid] |
|
14 =
|
[lactate]
[lactic acid] |
There is 14 times as much lactate as lactic acid at pH 5.
Question 2: What happens if 2 ml of 100 mM HCl is added to 100 ml of a 10 mM solution of phosphoric acid at pH = 7?
Answer:
This is a more involved problem. The first thing to realize is that phosphoric acid is polyprotic, meaning it has more than one H+ to donate, and therefore more than one pKa.
|
pKa1
|
|
pKa2
|
|
pKa3
|
|
|
H3PO4
|
 |
H2PO4- + H+
|
 |
HPO4-2+ H+
|
 |
PO4-3 + H+
|
Since this problem is at pH = 7, it is the pKa2 value that is important, for this is the pKa nearest the pH.
First we need to find the ratio of [HPO4-2]/[H2PO4-]:
Again, use the Henderson-Hasselbalch equation:
7 = 7.2 + log[HPO4-2]/[H2PO4-]
0.63 = [HPO4-2]/[H2PO4-]
0.63[H2PO4-] = [HPO4-2]
We know we have a total of
(0.01 moles/L) x (0.1 L) = 0.001 moles phosphoric acid
that at pH = 7 is made up of HPO4-2 and H2PO4-. ( Note 10 mM = 0.01 moles/L and 100 mL = 0.1 L)
0.001 moles = 1 mmole = HPO4-2 + H2PO4-
We know from above that [HPO4-2] = 0.63[H2PO4-]
so 1 mmole=0.63[H2PO4-] + [H2PO4-] = 1.63[H2PO4-]
[H2PO4-] = 0.6 mmoles
and so then [HPO4-2] = 0.4 mmoles
So far, this problem is much like the previous one. Now let's answer the question about the addition of 1 ml of 0.1 M HCl. From the high pKa2 value, we know that phosphoric acid is a weak acid. It will react completely with the HCl (which is the same as H+ in solution):
|
pKa3
|
|
|
HPO42- + H+
|
 |
H2PO4-
|
By adding 2 ml of 0.1 M HCl, we are adding:
(1 x 10-3 L) X (100 mmoles/L) = 0.2 mmoles H+
If we set up the grid as shown above,
| Reaction |
HPO4-2 |
H+ |
H2PO4- |
|
Initial
|
|
|
|
|
Change
|
|
|
|
|
Change after equilibrium
|
|
|
|
What is the pH of the phosphoric acid solution after the addition of HCl? Again, use the Henderson-Hasselbalch equation to solve
pH = 7.2 + log(0.6/0.4)
pH = 7.37
Note that this is not a big change. This is because of phosphoric acid is a buffer near its pKa and as such is able to resist changes in pH.
|



