|
||||||||||||
|
|
||||||||||||
Thermodynamics ReviewOverviewThermodynamics - it is an impressive term that might seem more than just a little intimidating at first. Luckily, like many things, once you get to know it a bit, it’s not as mysterious and difficult as it seemed at first. It’s really all about energy. The word "thermodynamics" comes from Greek roots meaning heat (thermo) and energy, or power (dynamics). So, Thermodynamics is really just the study of heat and energy, and how it relates to the matter in our universe. Thermodynamics is used in many fields of study, such as physics and engineering, to understand physical processes. Not surprisingly, it is also used by biochemists to understand processes and chemical reactions that occur in living organisms. Despite all these applications, the basic tenets of thermodynamics can be stated in just a few laws. There are three laws of thermodynamics, the first two of which are of most interest to biochemists. The third law, which has to do with properties of matter at a temperature of absolute zero (about minus 273 °C), won’t be discussed here. The First Law of ThermodynamicsThis is the law of the conservation of energy. It states that energy can neither be created, nor can it be destroyed. This means that the total amount of energy in the universe always remains conserved, or constant. However, energy can be changed from one form to another. There are many different forms of energy, some of which may be more useful than others for a particular process. Electrical, chemical, and mechanical are all examples of different forms of energy. The definition of energy is the ability to do work. Thus, with energy, biochemical reactions, the stuff of life, can proceed. The Second Law of ThermodynamicsThis is the law of increasing entropy. It states that the entropy of the universe increases with every physical process (change) that occurs. Entropy refers to the level of disorder, randomness, or chaos, of a system. The higher the randomness of a system, the higher its entropy. The more organized a system, the lower its entropy. A "system" is the part of the world we are interested in. It can be very small, like a single molecule, or as large as the entire universe.
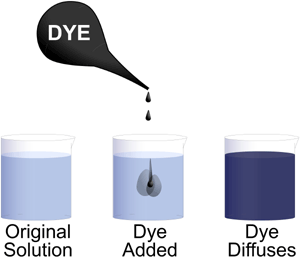
Entropy at work. A drop of dye placed in a cup of water will eventually result in an evenly colored solution, even if we never stir the liquid. The dye molecules distribute as evenly as possible throughout the volume of water.
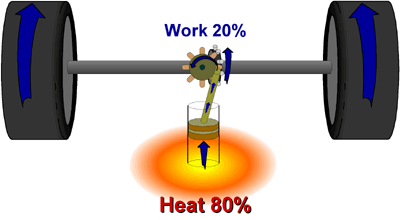
1. Naturally occurring, or "spontaneous" processes will always proceed towards the state that has the least potential energy. A good example of this is water flowing downhill. 2. Spontaneous chemical reactions must increase the disorder in the universe. We know from the first law of thermodynamics that energy cannot be lost, but it can change forms. What is lost, when a reaction is finished, is energy that is useful for doing more work. Some energy is always wasted in the form of heat. This is nature’s "heat tax."
This idea that entropy is always increasing can be a bit confusing. The second law states that the entropy of the universe must increase with each process. However, this is not the same as saying the entropy of a system must increase with each process. Remember, a system is the part of the world we are interested in. The system can become more ordered, but the price is that the surroundings must become much more disordered, so that there is still an overall increase in entropy for the universe. The human body is a case in point. Each person develops from a fertilized egg, which is just one cell. This zygote then develops into an embryo, and eventually into a full-grown human being, a highly ordered organism with many different organs and tissues. Thus, as it develops, the human body as a system becomes more ordered, and the entropy of that system decreases. We achieve this order through the ingestion of food (also ordered plant or animal tissue), which we break down through digestion into disordered waste. Our bodies also generate lots of heat, which we release into our surroundings, creating more disorder in the air molecules around us. (Now you know why your mother always made you wear that hat in the winter!) The laws of thermodynamics are useful because they can tell us whether any process is actually physically possible. Let’s see how this helps us understand biochemical reactions important to life. |
 |
Copyright 2006, John Wiley & Sons Publishers, Inc. |
 |
 We all have plenty of experience with the second law of thermodynamics in action. When a hot pan is placed in a sink of cold water, the pan cools down and the water warms up. The heat energy is more randomly distributed between the water and the pan. We would not expect the pan to get hotter while the water freezes. We know that when we accidentally knock over that expensive Ming Dynasty vase at the museum, it will break. As much as we wish that the random motion of the air around the pottery shards would force the vase back together and up on its pedestal, it just won’t happen. In these processes, the entropy of that system has increased. We never see hot pans freezing water, or broken vases magically mending themselves, because that would require a spontaneous increase in organization of molecules, or energy.
We all have plenty of experience with the second law of thermodynamics in action. When a hot pan is placed in a sink of cold water, the pan cools down and the water warms up. The heat energy is more randomly distributed between the water and the pan. We would not expect the pan to get hotter while the water freezes. We know that when we accidentally knock over that expensive Ming Dynasty vase at the museum, it will break. As much as we wish that the random motion of the air around the pottery shards would force the vase back together and up on its pedestal, it just won’t happen. In these processes, the entropy of that system has increased. We never see hot pans freezing water, or broken vases magically mending themselves, because that would require a spontaneous increase in organization of molecules, or energy.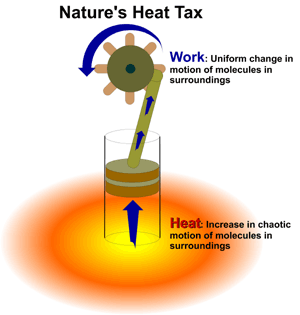 There are two important implications of the second law for studying biochemical reactions:
There are two important implications of the second law for studying biochemical reactions: