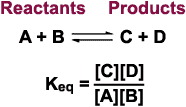|
||||||||||||
|
|
||||||||||||
Thermodynamics ReviewRelationship of the equilibrium constant to Gibbs free energyLet’s look at our hypothetical reaction from the discussion on Keq again:
If the reaction above proceeds so that at equilibrium, the concentration of products is greater than the concentration of reactants, then the reaction is said to lie "to the right." In that case, the concentrations of C and D will be higher than the concentrations of reactants A and B, and the Keq will be greater than 1. Remember from our look at the Gibbs Free Energy, that if the formation of products is favored, then the DG is negative. On the other hand, if the reaction is at equilibrium when there are still more reactants left over than products (lies "to the left"), then the concentrations of A and B will always be larger than the concentrations of C and D, and the Keq will be less than 1. Again, from our look at Gibbs Free Energy, when the formation of reactants is energetically favored, the DG is positive. Thus, there is a relationship between Keq and DG.
There is an equation that relates Gibbs Free Energy to the Equilibrium Constant: DG = -2.3RT log Keq R is a constant value (the gas constant, 8.3 J/mol/K ) For example, Keq is 4.692 x 103 for the reaction: a-Ketoglutarate + NAD+ + CoASH To calculate the DG at 25 °C (298 K), use the formula:
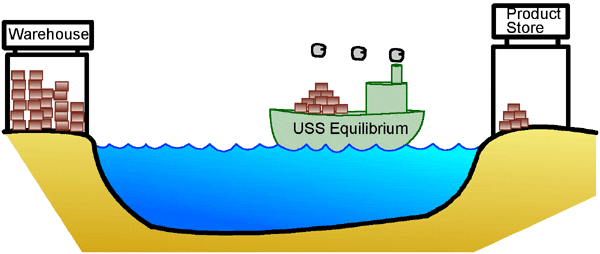
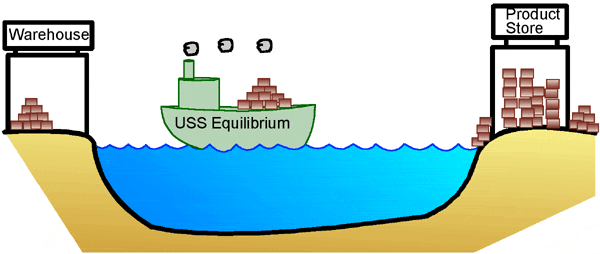
Why is all this important? The idea that there is a relationship between the concentrations of the reactants and products and the direction in which a reaction proceeds has important consequences for chemical reactions. Many metabolic reactions in our bodies (reactions that produce energy, or create building blocks to build up our bodies) can go forward or backward, depending on the surplus of reactants, or the demand for their products. Let’s use an analogy to make this important point a little more clear. Let’s say there is a small store that sells products on one shore of a lake. Because it’s small, the store owner keeps extra inventory in a warehouse on the other side of the lake. If the warehouse is full but the store is empty, the boat moves from the warehouse to the store, so that there is more product to sell. This is similar to a biochemical reaction proceeding in an environment where the reactant-to-product ratio is greater than it is at equilibrium. In other words, there is too much reactant (stuff at the warehouse), and not enough product (at the store). The reaction (boat) spontaneously proceeds toward equilibrium, which in this case is "to the right."
On the other hand, if it’s right after Christmas and suddenly there are a lot of returns, there is too much product at the store. The owner has to send some back to the warehouse. This is similar to a situation where a biochemical reaction is proceeding in an environment where the reactant-to-product ratio is smaller than at equilibrium. In other words, there is too much product while the warehouse sits empty. Again, the reaction moves spontaneously towards equilibrium, but in this case, that means the boat goes in the opposite direction, "to the left."
In our analogy, the store owner would ideally always have just enough product at the store to do a good business, with the rest stored at the warehouse. He constantly compares his ideal to the real situation, and adjusts the movement of his boat accordingly, bringing product in when needed, and shipping it back when there is too much at the store. Similarly, our metabolism can react to changes in environment, building up reserves or generating more energy for our bodies in response to our needs. This process of always trying to get back to equilibrium is known as Le Chatelier’s Principle. One last thing to remember; the word "spontaneous" sure makes it sound like these reactions happen fast. But the truth is, even when a reaction is thermodynamically possible, (has a negative DG value), it often happens very slowly. This is especially true of many biochemical reactions that occur under physiological conditions, that is, within our bodies. Think of what would happen to our store owner if his boat were to break down. He might try to float his packages across the lake, but it could take forever for them to get to the other side. So, even if the warehouse is full, the store will get little or no product unless the boat moves. Enzymes act in much the same way in biochemical reactions. Metabolic reactions in our bodies are catalyzed, or helped along, by special proteins called enzymes. They greatly speed up the rate at which reactions occur, so that reactions that are thermodynamically possible but very slow can now proceed at a rate that makes them useful for sustaining life. |
 |
Copyright 2006, John Wiley & Sons Publishers, Inc. |
 |